Category B Infectious Substance Packaging Instructions Category B infectious substances are infectious but do not meet the criteria for Category A (ie, they are not capable of causing permanent disability, lifethreatening, or fatal disease when exposed to humans or animals)Guide to Packaging Category B Diagnostic Samples You can learn more about the packaging system and its componentsby following these 123 Packaging Instructions* STEP 1 Primary Receptacle Package in primary receptacle Leakproof (for liquids) or sift proof (for solids) Packaging Category B Infectious Substances A material that is classified as a Category B infectious substance and that meets the definition in a2 must be triplepackaged, meeting the packaging requirements in 49 CFR , and sent as FirstClass Mail, Priority Mail, or Express Mail

Packaging Demonstration Category B Infectious Substances Youtube
Category b infectious substance packaging
Category b infectious substance packaging-Packaging and documents are prepared by a person with awareness of category B shipping requirements Triple packaging system is employed with primary specimen container, secondary packaging, and outer packaging according to IATA and DOT requirementsCategory B An infectious substance which does not meet the criteria for inclusion in Category A Infectious substances in Category B shall be assigned to UN3373 Diagnostic specimens, assigned to UN 3373, are human or animal materials that are being transported only for the purpose of diagnosis or investigation



Biological Substance Category B Label Trovoadasonhos
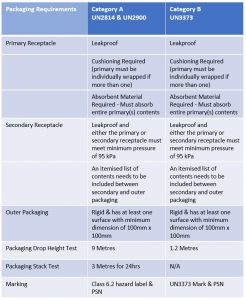
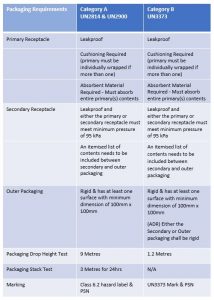
Substance, Category B" and the identification number UN3373 Packaging Category B infectious substances must be tripled packaged and compliant with IATA Packing Instruction 650 detailed in Figure 2 The maximum quantity for a primary receptacle is 500 ml or 500 g and outer packaging must not contain more than 4 L or 4 kg Labeling The outerCategory B infectious substances must be tripled packaged and compliant with IATA Packing Instruction 650 detailed below The maximum quantity for a primary receptacle is 500 ml or 500g and outer packaging must not contain more than 4 L or 4 kgCategory A (P6) UN 2814 Infectious Substance affecting Humans UN 2900 Infectious Substance affecting Animals Only Category B (P650) UN 3373 Biological Substance, Category B Exempt Exempt Human Specimen Exempt Animal Specimen P6 P954 P650 P954 Minimal triple packaging P954 Shipper must be trained (dry ice is a dangerous good)
The proper shipping name "BIOLOGICAL SUBSTANCE, CATEGORY B" in letters at least 6 mm high shall be marked on the outer packaging adjacent to the diamondshaped mark DfT interpretationPricing and Availability Saf T Pak Inc STP300 Category A Frozen Insulated Shipping System (Overpack for UN 2900, UN 2814), 4/Case STP300 Overpack, Category A Shipping; Comparison of Category A, B, and Dry Ice Packing Comparison of Category A, B, and Dry Ice Packing Page 1 Last Updated Category A Packing Instruction 6 Category B Packing Instruction 650 Packaging 1 Leak proof primary receptacle(s) Maximum volume 50ml (50g solid) Leak proof primary receptacle(s) Maximum volume 1Liter (4 Kg
Category B Packaging Requirements The basic triple packaging system is used with the following specifications Inner packaging comprising a watertight (leakproof) primary receptacle (ie specimen container) o Maximum quantity 1 litre (for liquids) or 1 kilogram (for solids)Packaging and documents are prepared by a person with awareness of category B shipping requirements Triple packaging system is employed with primary specimen container, secondary packaging, and outer packaging according to IATA and DOT requirementsCategory B packaging requires a marking (or label) as indicated below in accordance with IATA Packing Instruction 650 requirements Part No (3 1/2″ x 4″) IATA label for Biological Substance, Category B (click here for other shipping labels)



Azdhs Gov Documents Preparedness State Laboratory Category A And B Shipping Examples Pdf




Iata S Packing Instructions 650 Environmental Health And Safety
Packaging developed in accordance to the regulations set forth by the USDOT, Transport Canada, ICAO and IATA Biological substances are assigned to one of the following categories Category A Infectious substance, Category B Infectious substance, Exempt patient specimens, Biologic not regulated for transport(b) Samples of each packaging must be prepared as for transport except that ntifreeze Each primary receptacle must be filled to 98 percent capacity Category B Infectious Substance Package, label, and ship low or moderaterisk specimens as a Category B infectious substance (UN 3373) in accordance with the US Department of Transportation's Hazardous Materials Regulations and the International Air Transport Association Dangerous Goods Regulations



Category B Biological Shipper 9 X 4 X 4 Icc Compliance Center




Online Self Study Shipping Of Infectious Substances And Other Biomedical Materials Annual Update Ppt Download
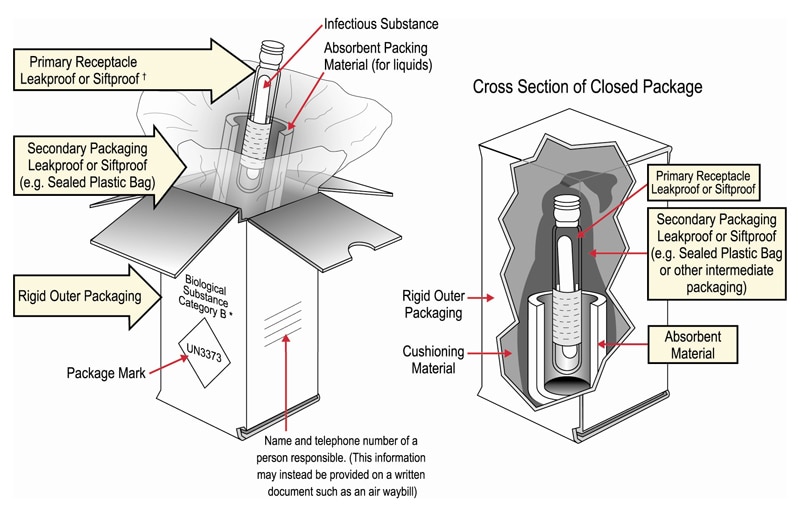
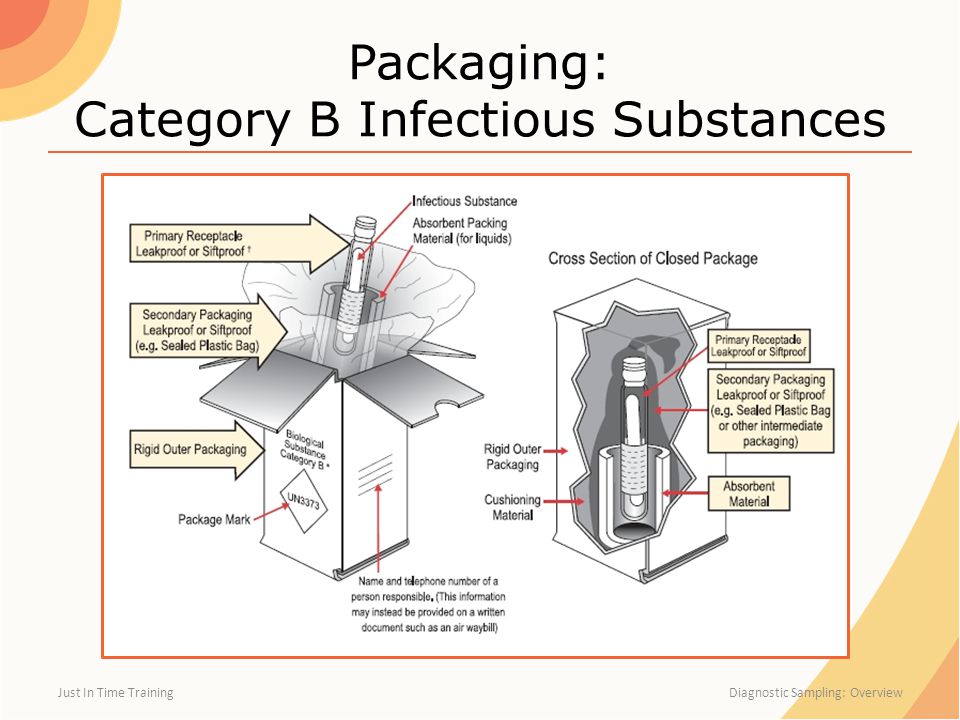
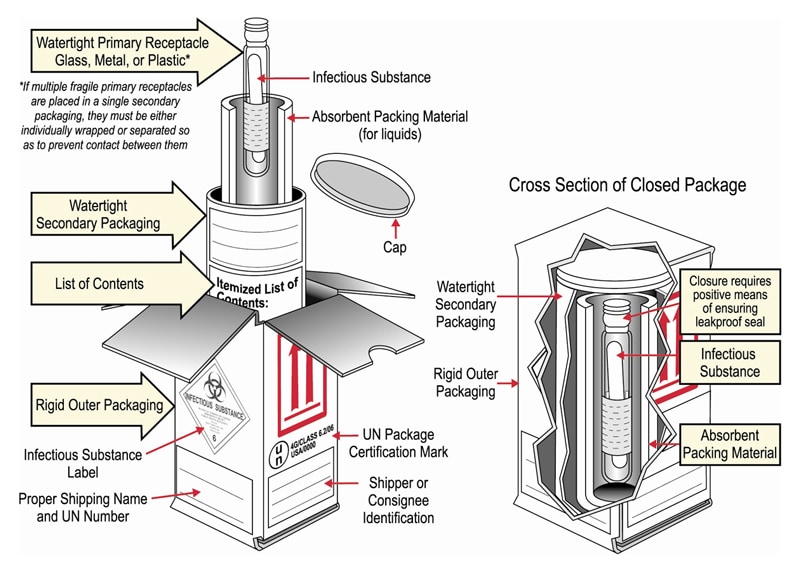
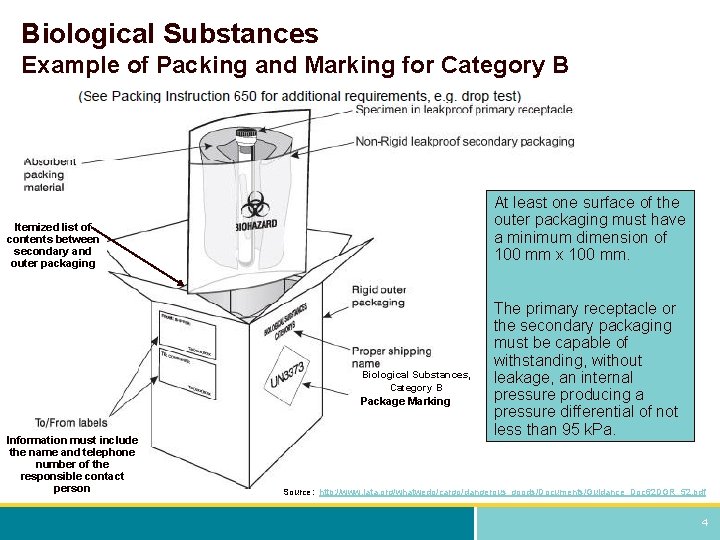
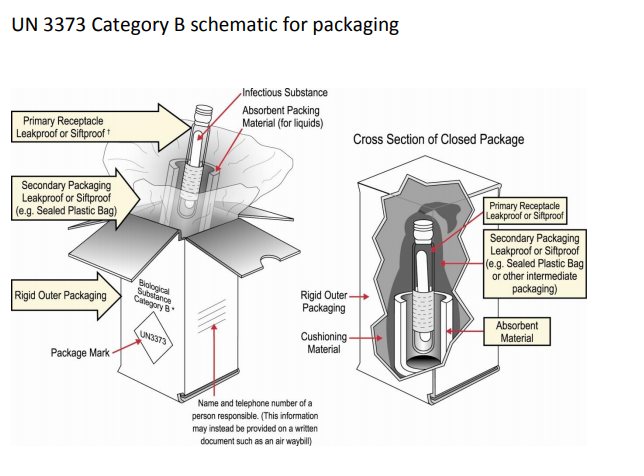
Packaging DIAGRAM OF CATEGORY B PACKAGE CROSS SECTION OF CLOSED PACKAGE Name and Telephone Number of a Person Responsible (This Information May Instead be Provided on a Written Document Such as an Air Waybill) Biological Su bstanc e Category B* Primary Receptacle Leakproof or Siftproof Secondary Packaging Leakproof or Siftproof (eg Sealed3021 Occupancy Classification Occupancy classification is the formal designation of the primary purpose of the building, structure or portion thereof Structures shall be classified into one or more of the occupancy groups listed in this section based on the nature of the hazards and risks to building occupants generally associated with theFor solids, the secondary packaging must be siftproof These illustrations below are not intended to represent




Category B Biological Substance Frozen Insulated Shipper Exempt System Case Of 8 Labelmaster




Ppt Online Self Study Powerpoint Presentation Free Download Id
Category B Ambient IATA Packaging Category B Ambient 95kPa Bag, 6Tube The ambient transport system for blood and urine tube transport includes 95kPa bag, AquiPak™ segmented absorbent pouch, cushioning material, list of contents card, biohazard symbol label, Biological Substance Category B label, and medium transport box (ID 7 x 4 x 4Step 3 Packing Category A and B and Exempt Human and Exempt Animal Specimens Job Aid Use the pages below as a reference for packing Category A, B, and Exempt Specimens Category A Substance Packaging NOTE The packaging is the same for both types (UN 2814 and UN2900) of Category A packaging, only the UN mark and Proper Shipping Names change Infectious Substances that fall in both Category A and Category B (excluding UN3291) require triple packaging;



Www Kdheks Gov Labs Downloads Chlamydia Packaging Handout Pdf




Complete Packaging For Un3373 Bio 0502 Sb Serpac
"An infectious substance which does not meet the criteria for inclusion in Category A Infectious substances in Category B must be assigned to UN 3373" (Dangerous Goods Regulations, ) Note The proper shipping name of UN 3373 is Biological substance Category BCustomers who ship Biological Substance, Category B (UN 3373) shipments must comply with local, state, and federal laws governing identification, classification, packaging, and package markings (which may be in label form) FedEx Express strictly adheres to the IATA, ICAO, and US government guidelines for materials categorized as Biological Substance, Category B (UN 3373)Category B Biological substances need to packed in way that prevents loss of contents that may occur due to vibration, changes in temperature, humidity or pressure Find out what packaging materials you need, how to pack the substances securely and label the box correctly



Ehs Usc Edu Files Shipping Guidelines Pdf




Therapakbiological Substance Category B Ambient Shipping Systems Mailing Fisher Scientific
Category B" in letters at least 6mm high must be marked on the outer packaging adjacent to the diamondshaped mark Unless all package markings are clearly visible, the following conditions apply when packages are placed in an overpackStep 2 PACKAGING and DOCUMENTATION Pack and Ship Clinical Specimens as Category B Infectious Substances BLOOD SPECIMENS URINE SPECIMENS BLOOD TUBE (NONGEL) • 3ml or larger purpletop • 3ml or larger gray/greentop URINE CUP • Sterile, plastic, screwcapped • Keep blood tubes separated, or wrap tubes to prevent contact between themOuter box OD 155 x




Example Of The Triple Packaging System For The Packing And Labelling Of Download Scientific Diagram



Biological Substance Category B Label Trovoadasonhos
required absorbent included w/ cushioning for fragilesTamperEvident Packaging b) Citizen petition requirements ¨ Name of DP or drug class with a list of DPs within the class ¨ Reasons why compliance is unnecessary or cannot be achievedCategory B infectious substances and cultures must be packaged using the IATA/DOT Requirements for Packing Instructions (PI) 650 WARNING Using the incorrect packing materials, package, and labels can cause the package to be out of compliance Labels The following labels must be on the vertical side of the outer package when shipping




850 Ml Bio Bottle Kit Case Of 18 By Asc Inc



Www Pace Edu Sites Default Files 21 05 Biological Substance Category B Shipping Fact Sheet Pdf
(1) A Category B infectious substance must be packaged in a triple packaging consisting of a primary receptacle, a secondary packaging, and a rigid outer packagingPackaging and package markings (which may be in label form) FedEx Express strictly adheres to the IATA, ICAO and US government guidelines for materials catagorised as Biological Substance, Category B (UN 3373) General Packaging Requirements For Biological Substance, Category B (UN 3373) shipments,Ambient UniPak Category B For those needing protection for the sample, significantly increased capacity and reusability, EXAKT's UniPaks are ideal The UniPak containers come in 4 sizes The outer packaging consists of a corrugated box preprinted with the Biological Substance, Category B and UN3373 labels, a cardb




Packaging For Biological Substance Category B Un3373 Dg Consulting



Specialized Packaging For Your Shipments Fedex United Kingdom
Packaging of Infectious Biologicals Category BThis consists of Leakproof primary receptacle (s)UN3373 mark, Biological Substances, Category B, Name, and Phone number of a responsible person UN3373 mark, Biological Substance Category B UN specification packaging Must meet the criteria of Packing Instruction 650 (see below for package criteria) Type P6, or




Biological Substance Category B Extreme Ambient Shipper Kit 4 Case Transmed Company




Shipping Infectious Substances
Category B Substances Maximum 4 litres or 4 kg per package for passenger or cargo aircraft Maximum 1 litre per primary container for passenger or cargo aircraft There are no limitations for shipments by road, rail or seaFor Biological Substance Category B (UN 3373), enclose an itemized list of contents between the secondary packaging and the outer packaging For liquids, the secondary packaging must be leakproof;Biological substances need to packed in way that prevents loss of contents that may occur due to vibration, changes in temperature, humidity or pressure We'



Www Ehs Washington Edu System Files Resources 09 Pi650 Inst Pdf




Biological Substances Packaging For Cold Chain Australia
Packaging suitable for Category B, UN3373 Biological Substances We have a range of UN packaging solutions for Class 62 Infectious Substances within Category B (goods classified under UN3373 Biological Substance, Category B), which must be packed under packing instruction 650 – found within IATA, ADR & IMDG dangerous goods regulationsCategory B Infectious Substances (USPS) Proper Shipping Name and ID Number All Category B infectious substances must meet the triple packaging requirements of 49 CFR and be sent as Express Mail, Priority Mail, or FirstClass Mail service The proper shipping name Category B infectious substances is Biological substance, CategoryFor use with SafTTemp products or Dry ice for thermal control;




Pointers On Shipping Clinical Samples Biological Pages 1 6 Flip Pdf Download Fliphtml5




Therapakrefrigerated Biological Substance Category B Shipping Systems Mailing Fisher Scientific
432 PSN "Biological Substance, Category B" 433 Overpack if applicable 44 Documentation compliance 441 Mandatory statement on airway bill (connote) 442 Number of packages (connote) 443 Itemized list of contents (inbetween secondary packaging & outer packaging 45 For refrigerated or frozen UN3373 shipmentsThe outer package must be clearly and durably marked with the words "Biological Substance, Category B" It also needs to be marked with a diamond with sides of at least 50 mm (2 inches) and a line thickness of at




Shipping Biological Substances Category B Ppt Download




Category B Biological Substance Insulated Shipper Large Case Of 8 Labelmaster



Www Cdc Gov Coronavirus Mers Downloads Lab Un3373 Packaging Schema Pdf




Online Self Study Shipping Of Infectious Substances And Other Biomedical Materials Annual Update Ppt Download




Un3373 Category B Shipping Kit



Www Mayocliniclabs Com It Mmfiles Infectious Specimen Shipping Guidelines Pdf



Http Www Kdheks Gov Labs Downloads Virus Pictorial Guide Pdf




Class 6 2 Biological Substance Category B Un3373 Hazard Label 50mm X 50mm Air Sea Containers Us



Ehs Unl Edu Sop S Ship Biological Substances 7 Pdf




95 Kpa Biohazard Bag Iata Packaging Category B Ambient Therapak




Category B Environmental Health And Safety Business Operations And Facilities




Biological Substance Packaging Un3373 Category B Packaging




Categorie Infectious Substances Packaging



Www Ehs Washington Edu System Files Resources 09 Pi650 Inst Pdf



Infectious And Biological Substance Category A B Packaging Air Sea Containers Compliance Blog



Packaging And Transporting Infectious Substances Smallpox Cdc
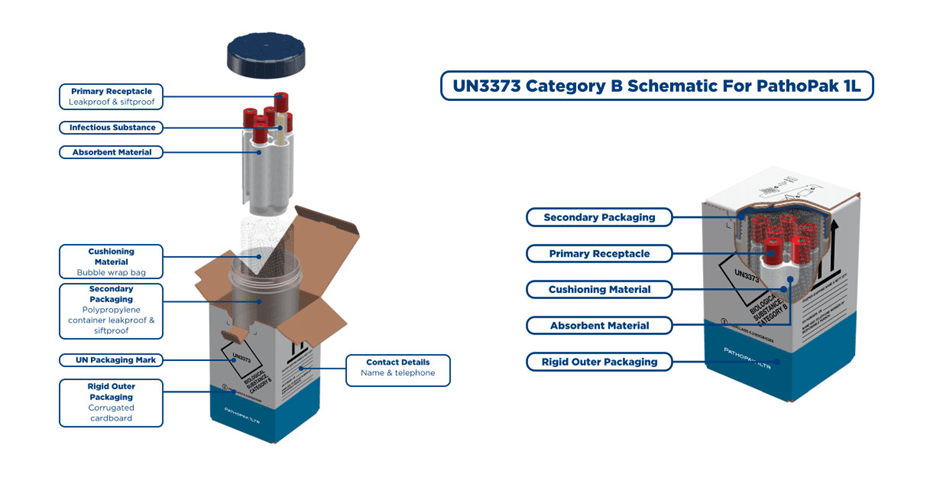



Un3373 Compliance A Simple Guide Intelsius Cold Chain Solutions




Packaging And Shipping Sarsco V2 Specimens Cultures Isolates



Www Cdc Gov Coronavirus Mers Downloads Lab Un3373 Packaging Schema Pdf




Packaging Demonstration Category B Infectious Substances Youtube




Shipping Biological Substances Category B Ppt Download



Http Www Nj Gov Health Phel Documents Cat B Infe Sub Pdf




Pathopak Intelsius Cold Chain Packaging Solutions



Staff Ki Se Sites Default Files Migrate How To Know Whether Your Sample Is Classified As Dangerous Goods 2 Pdf



Infectious Substances Packaging Explained Air Sea Containers




Biological Substance Category B Un3373 Packaging And Labeling For Air Transport Quick Specialized Healthcare Logistics



1



Infectious Substances Category A Category B Packaging Explained Air Sea Containers Us




Biological Substance Category B Packaging Download Scientific Diagram



Collection Packaging Shipping Overview Ppt Download




Iata Un3373 Biological Substance Category B Labels Shipping And Compliance Labels Therapaka Case Of 500 Amazon Com Industrial Scientific




Category B




Packaging




Online Selfstudy Shipping Of Infectious Substances And Other




Medimail International Medical Products




Biological Substance Category B Ambient Shipper Kit 6 Case Transmed Company



Azdhs Gov Documents Preparedness State Laboratory Category A And B Shipping Examples Pdf



Http Nih Dmsc Moph Go Th Km Safety19 01 09 1 Packaging Shipping Of Hazardous Pdf




Un3373 Category B Packaging Bioshipper 1 Code 761 Contact Isovation




Temperature Safe Shipping And Transportation Packaging Category B Infectious Substances Packaging Models Polar Tech Industries



Mmcri Org Deptpages Em Downloads Gcp Sop 1111c Pdf



Azdhs Gov Documents Preparedness State Laboratory Category A And B Shipping Examples Pdf



Category B Shipping Label Juleteagyd



Www Albertahealthservices Ca Assets Wf Lab Wf Lab Biological Substances Category B Iata Packing Instructions P650 Jobaid Pdf



Www Nyu Edu Content Dam Nyu Environmentalhealthsafety Documents Category b Pdf



Ehs Unc Edu Wp Content Uploads Sites 229 04 Shipping Biological Materials Manual Pdf




Dot Hazardous Materials Flashcards Quizlet



1



Www Bu Edu Ehs Files 04 Shipping Biologicals Training Pdf



Packaging And Transporting Infectious Substances Smallpox Cdc



Www Doh Wa Gov Portals 1 Documents Pubs 302 024 Categorybshipping Pdf




Shipping Dangerous Goods Un3373 Biological Substance Category B Purolator



Www Icao Int Safety Dangerousgoods Dgp working papers Dgp Ip 007 Pdf



Www Nyu Edu Content Dam Nyu Environmentalhealthsafety Documents Category b Pdf



Prd Medweb Cdn S3 Amazonaws Com Documents Infectioncontrol Files 1 Covid 19 Specimen Submission Guide 03 04 Pdf




Category B Infectious Substance Packaging Cleveland Clinic Laboratories



Processing Isc And Dry Ice In Campus Ship



News Mayocliniclabs Com N1 96ecea7b0de Uploads 19 07 Dangerous Goods Handout English Pdf
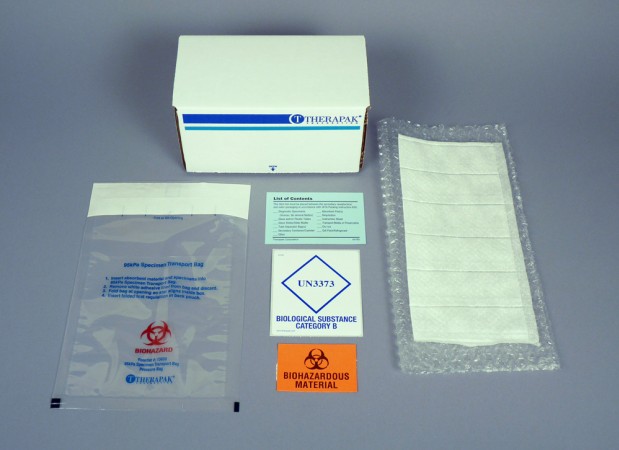



95 Kpa Biohazard Bag Iata Packaging Category B Ambient Therapak
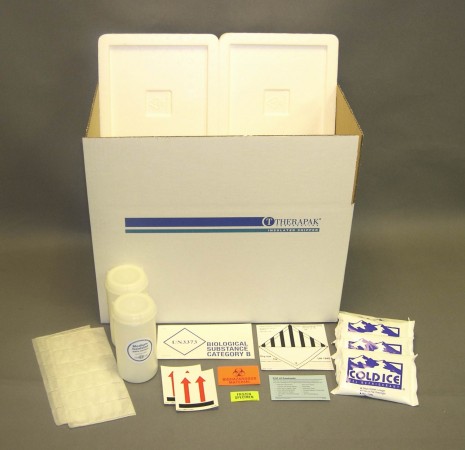



Category B Combo Online Product Catalog Therapak
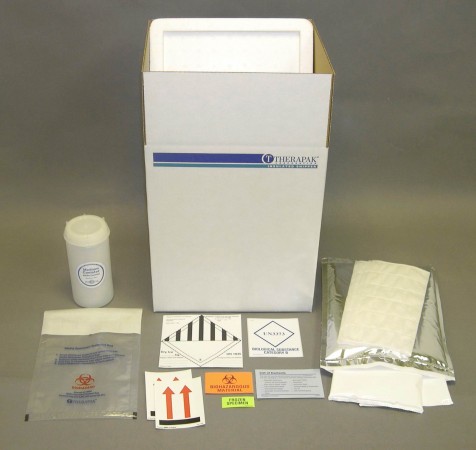



Category B Combo Online Product Catalog Therapak




Information For Dispatching Samples To Aru Biomarker Lab Cambridge Uk For Cortisol Analysis Aru Biomarker Lab



Intranet Birmingham Ac Uk Hr Documents Public Hsu Hsuguidance Guidance Note 5 Transport 15 Docx Pdf



Www Unmc Edu Ehs Chemical Safety Biologicalsdryiceshippingexamples Pdf



1



Static Compliancetrainingonline Com Docs Dot Transporting Infectious Substances Safely Pdf



Www Unmc Edu Academicaffairs Compliance Areas Export Control Ehs Docs Export Bisg Pdf




Un3373 Shuttlepac Shuttlebox Cost Effective Packaging




Catagory B Un3373 Postal Pack Air Sea Containers



Www Royalmail Com Sites Default Files Guidance Document Infectious Substances Pdf




Biological Substance Packaging Un3373 Category B Packaging




Infectious Substances Packaging Explained Air Sea Containers



Covid 19 Packaging And Shipping Environmental Health Safety




Pointers On Shipping Clinical Samples Biological Substance Category B Un 3373 And Environmental Test Samples Pdf Free Download




Complete Packaging For Un3373 With 95kpa Jar 0 5 L Serpac




Md8051v06k25 Exakt Pak Category B Ambient D Pak Insulated Shipping Inmark Life Sciences




Category B Infectious Substance Packaging Cleveland Clinic Laboratories




Temperature Safe Shipping And Transportation Packaging Polar Tech Industries Inc Category B Infectious Substances Packaging Models 06 Polar Tech Industries



Infectious Substance Transportation Introduction Triple Packaging And




Complete Packaging For Un3373 With 95kpa Tested Secondary Serpac



Azdhs Gov Documents Preparedness State Laboratory Category A And B Shipping Examples Pdf



1



0 件のコメント:
コメントを投稿